EVALUATION OF THE VIRUCIDAL ACTIVITY of ViruBLOC Nasal Microgel Spray, according to UNI EN 14476:2019 (modified)
Report N. 02709.21M00008 dated 19/04/2021 - Complife Italia Srl / LabAnalysis Srl
PURPOSE OF THE STUDY
The study was performed to evaluate the efficacy of the product VIRUBLOC NASAL MICROGEL SPRAY Ref. 2101134R6 in inhibiting the infecting capacity of an enveloped virus (lipid pericapsid), such as Vaccinia virus, strain Elstree ATCC VR-1549. The test method applied is taken from the UNI EN 14476:2019 standard, transposition of the EN14476:2013+A2 standard (July 2019 edition) and takes into account the corrections introduced on 24/07/2019.
CONCLUSIONS
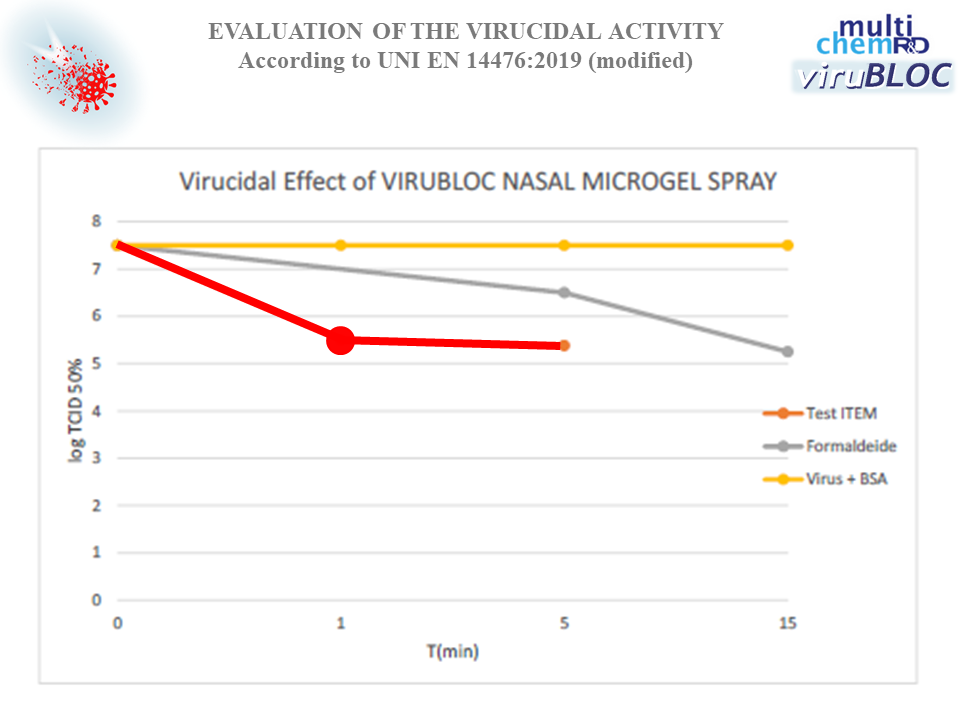
The product VIRUBLOC NASAL MICROGEL SPRAY REF. 2101134R6, tested at 100% concentration, positive control Formaldehyde 0.7%, showed virucidal activity vs. Vaccinia virus, strain Elstree ATCC VR-1549, as represented by the graph and as follows:
- Contact time 1 min: reduction of 2.00 ± 0.46 log TCID50 (Tissue Culture Infectious Dose = infecting capacity of the virus, expressed as the amount of virus needed to destroy or cause any other type of cytopathic effect (CPE) in 50% of infected cells or cultures).
- Contact time 5 min: reduction of 2.12 ± 0.25 log TCID50.